

The instructions for use developed by a medical device manufacturer commonly referred to as an IFU is an integral part of the process that produces properly decontaminated and sterilized instrumentation and implants for patients. These IFUs are very detailed and range from simple to very complex. Some IFUs can call for specialized processing equipment and specific times in sections of the process. Strict adherence to the guidance is required to properly prepare instruments and implants for surgical care and patient safety. The following guidance is from the Joint Commission regarding decontamination.

IFUs for Medical Instruments and Devices:
“…It is important to understand that each patient care item has its own IFUs for cleaning and disinfection and the expectation is that the organization will follow those instructions. Failure to follow such instructions or misuse creates significant risk to safe, quality care.”

IFUs for Cleaning, Disinfection and Sterilization Products:
“Products used during cleaning, disinfection and sterilization include specific IFUs to ensure efficacy and or confirmation that cleaning, disinfection or sterilization cycles are successful. Accredited organizations must follow instructions for quality control of the process, including dilution of products, efficacy testing of the solution or process, exposure times, and acceptable temperature and pressure ranges.”
With the two guidance references in mind, let’s explore the IFU world from the context of the time required and equipment needed to complete IFUs present in many of the total joint instrumentation systems. Also note that the American Joint Replacement Registry 2023 Annual Report indicates that 22% of revision total hip cases and 34% revision total knee cases are related to infection and inflammation diagnosis. While no assertions are made in the report about the cause of these events it is a stark reminder of a very common complication and risk to patients undergoing total joint surgery. The need to make every effort to provide processes that properly prepare the surgical suite and instrumentation for use must be a priority.
Decontamination
Decontamination is known to be the most important part of the process that prepares surgical instrumentation and implants for patient care. IFUs for decontamination of surgical equipment are specific to the instrument or group of instruments being processed. The goal of combining the manual inputs of hand washing, soak times and specific mechanical cycles for sonic treatments and washer decontamination units. The goal of these activities is to eliminate any bio-burden from the instrumentation. If the bio-burden is not removed at an extremely high level colonizing units can survive the final sterilization cycle and infect a patient.
Interacting with the IFU for the actual instrumentation are the various IFU requirements for the various supplies and chemicals that are used in the decontamination process. These IFUs for chemicals and cleaning devices are also quite detailed and per the Joint Commission guidance mentioned above must be followed to realize the goal of removing bio-burden from the instruments. All surgical facility policies require the adherence to these IFU requirements.
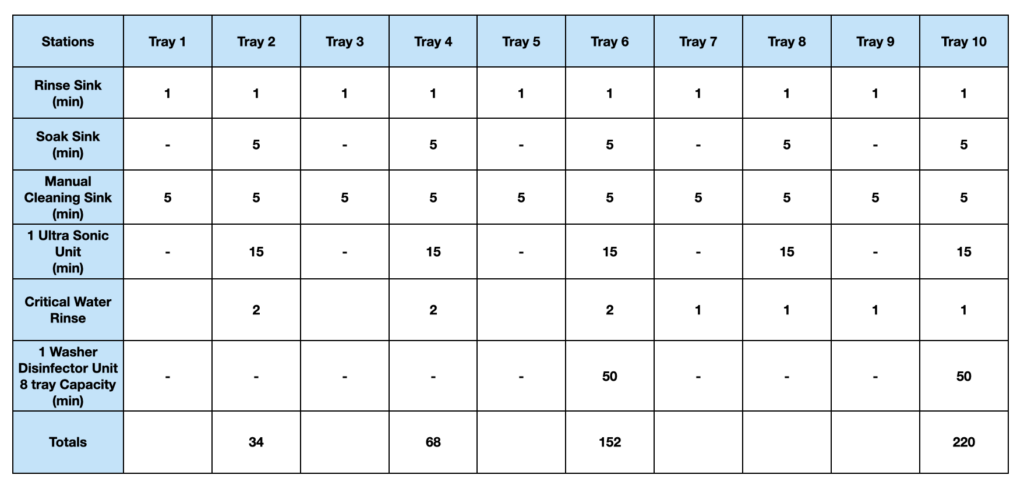
The grid below displays the task, equipment required and time required per station for a particular set of total joint instrumentation. This model assumes a 10 tray set of instrumentation with an average of 25 instruments per tray and all of the equipment needed to properly perform the IFU requirement. Times are the times per tray processed.
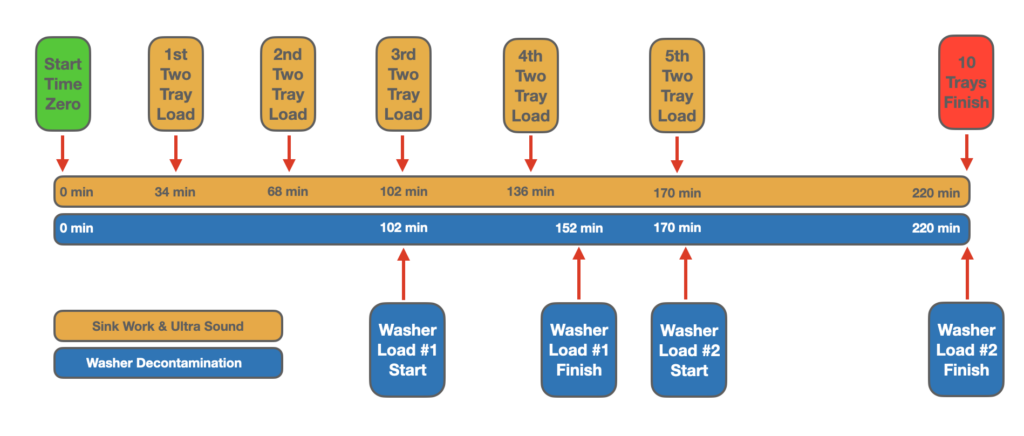
The timeline below provides a visual reference for the movement of the trays as they start and complete each station. The actual requirement via the IFU for the instrumentation will require 220 minutes assuming one washer and one ultra sound unit with the capacities mentioned on the grid.
Discussion
The increase in total joint procedures is straining the resources of ASCs. SPD departments in ASCs are typically designed and equipped for high turnover on smaller instrument trays and simple general instruments. As total joint procedures and other high acuity case move to the ASC setting decontamination IFU compliance challenges will grow. For example, most SPD departments in ASCs are small and many were not built with items that create efficiencies in the decontamination process such as three bay sinks. Some centers are lacking the sonic equipment that is commonly required by the instrument manufacturers IFUs on both the manual and mechanical processes referenced in their materials. Another challenge is the lack of redundancy of critical equipment. ASCs when properly equipped with washer decontaminate units and sonic units will typically have only one of each. Any maintenance issues with the machines results in a very difficult challenge. Opportunities to add additional equipment for processing are usually limited by the floor space of the existing department. Increasing the size of the department is a very expensive proposition and the only floor space must be captured from an area of existing use.
One part of the discussion that is usually understated as patients begin to chose total joint surgery in the freestanding ASC setting is the number of general trays that are needed for each surgery. Each total joint case usually needs retractor sets, power equipment and other ancillary trays in addition to the eight to twelve trays of vendor specific equipment required.
Enter the Sterile Processing Logistic Center (SPLC)
A Sterile Processing Logistics Center (SPLC) is a purpose built facility that is engineered and equipped to manage the sterile processing of instrumentation from the initial decontamination to a wrapped and sterilized state. These centers are specifically designed to process the most complex surgical instrumentation and follow the most difficult IFU process recommended by the instrument manufacturers. The SPLC has a redundancy of critical equipment and storage capabilities that allow processing to continue should any maintenance challenge with a single piece of equipment or work station arise.
Using an SPLC provides the ASC an opportunity to “Right Size” their existing department operations by moving processing and sterile storage of vendor total joint trays to the purpose built facilities. The ASC can now concentrate on the assets they own to increase the turnover and efficiency on the items those departments were designed to manage. The opportunity to increase the turn rates on the general trays is the most capital efficient model available to the ASC. This option saves on any remodeling needed to increase capacity or add equipment generally required by manufacturer’s IFUs or the need to purchase additional general trays and equipment to accommodate the lengthy processing schedules as total joint vendor trays hit the decontamination and sterilization lines at the facility.

SPDx Phoenix
Sterile Processing Express, LLC has opened its first of many SPLCs planned for the United States in Phoenix, AZ. The Phoenix facility is operational and will be servicing the surgical facilities in Phoenix and Tucson. Please direct inquiries for services and investment opportunities to:

